Vertex begins treating first patients with new SCD gene therapy
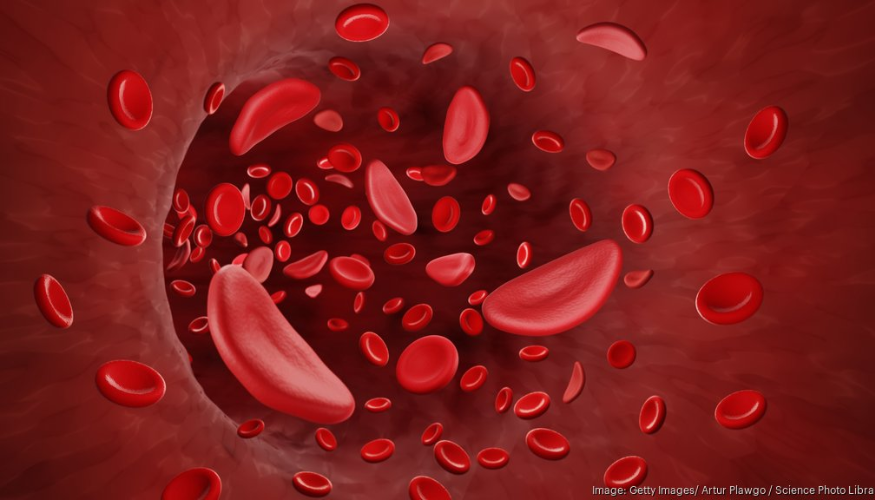
Recently, New England Council member, Vertex Pharmaceuticals, announced that its new CRISPR-based sickle cell treatment, Casgevy, has started being used on its first patients. On May 6, Vertex said that it had collected cells from five patients for the treatment, which is approved by the FDA for sickle cell and transfusion-dependent beta-thalassemia.
According to a press release from Vertex, Casgevy is a “non-viral, ex vivo CRISPR/Cas9 gene-edited cell therapy that has been shown to reduce or eliminate vaso-occlusive crises for patients with sickle cell disease and transfusion requirements for patients with transfusion-dependent beta-thalassemia.” It is a one-time therapy for patients 12 years and older. As of mid-April, Vertex has activated over 25 authorized treatment centers around the world for Casgevy.
When the treatment was approved by the FDA in December of last year, health professionals across the U.S. stressed the importance of the therapy. “I think this is a pivotal moment in the field,” said Dr. Alexis Thompson, chief of the division of hematology at Children’s Hospital of Philadelphia, who has previously consulted for Vertex. “It’s been really remarkable how quickly we went from the actual discovery of CRISPR, the awarding of a Nobel Prize, and now actually seeing it being an approved product.”
The New England Council commends Vertex for the continuation of this life-saving work.
Read more in the Boston Business Journal.